ABSTRACT
Background
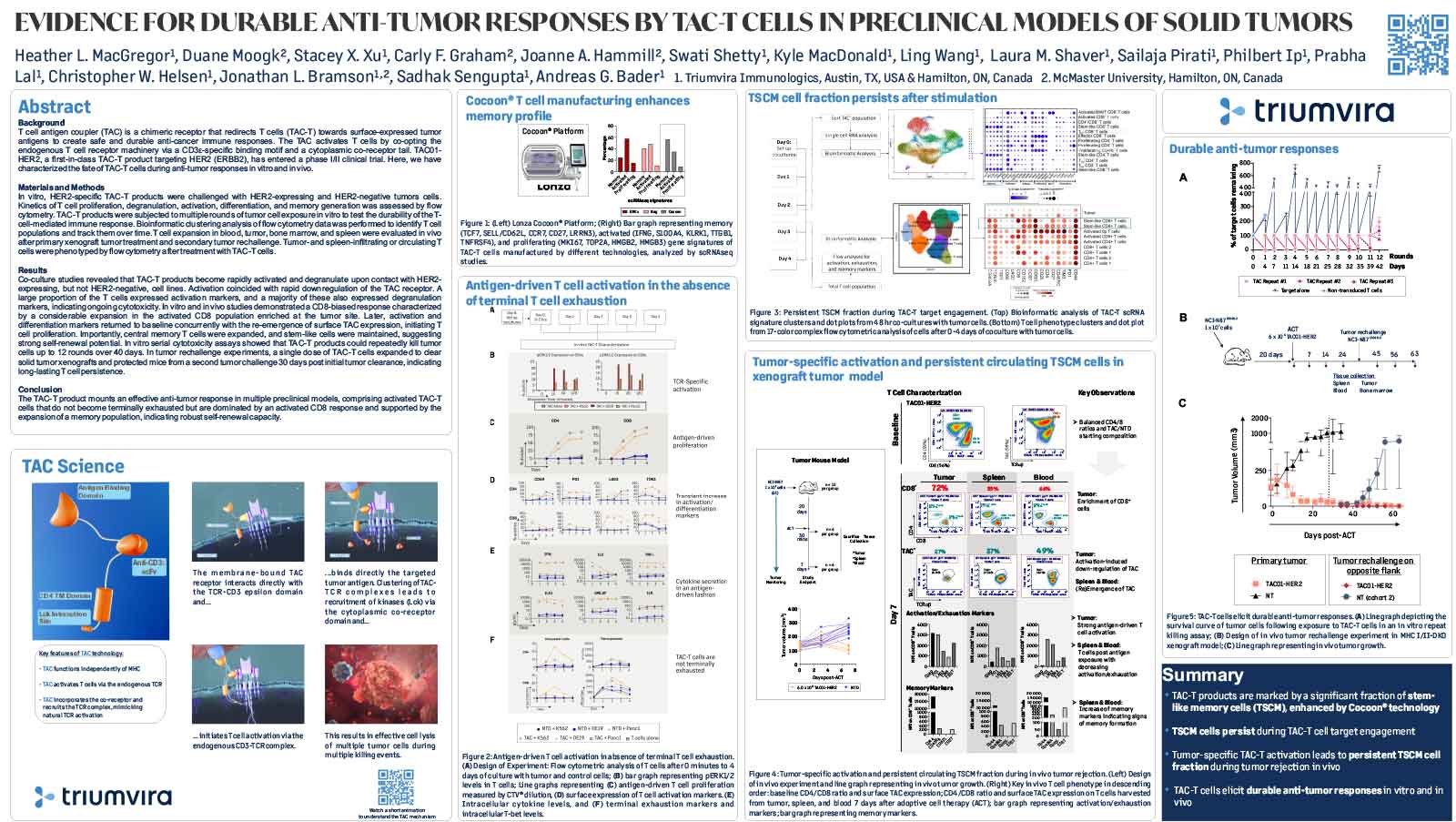
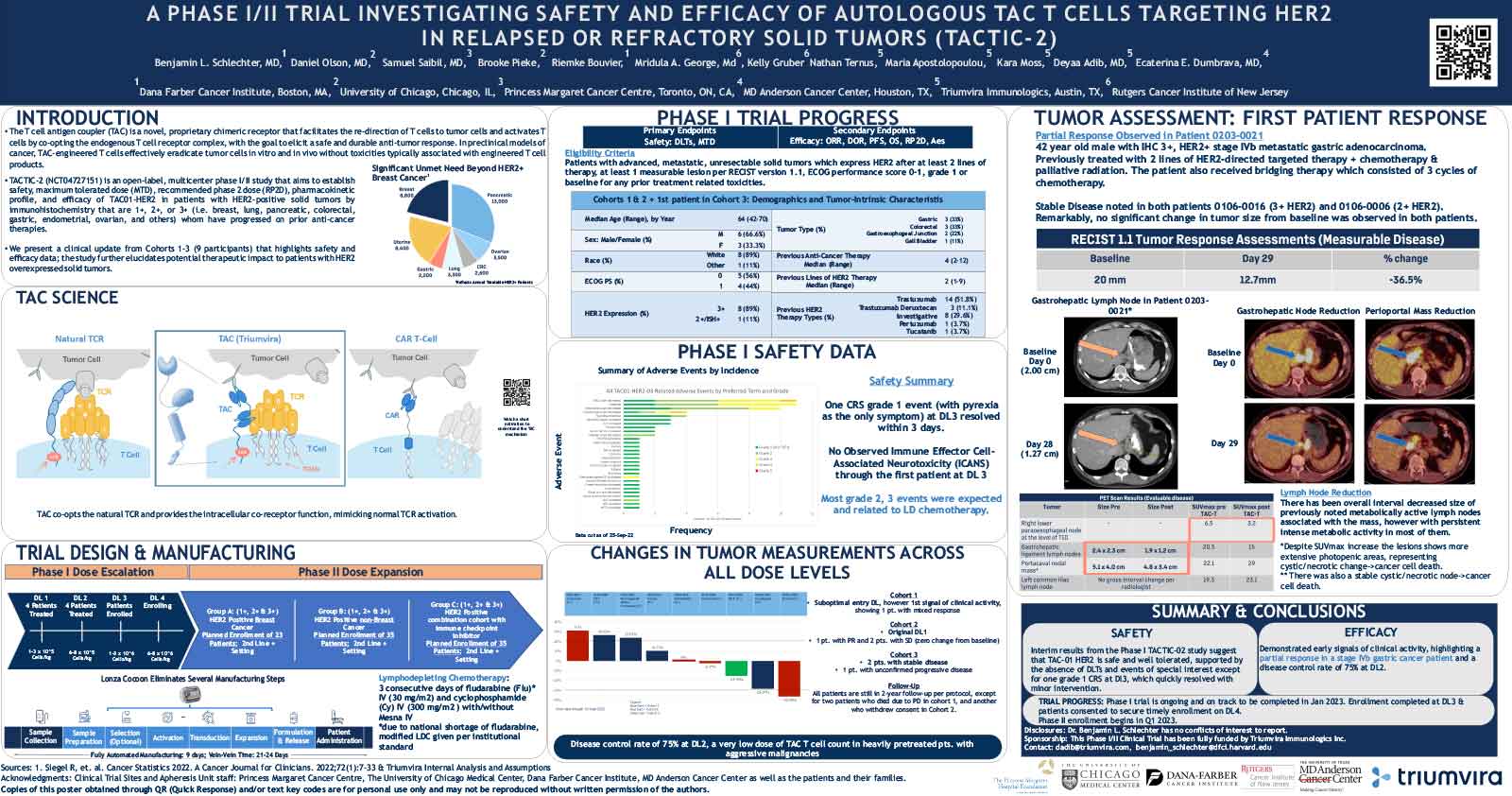
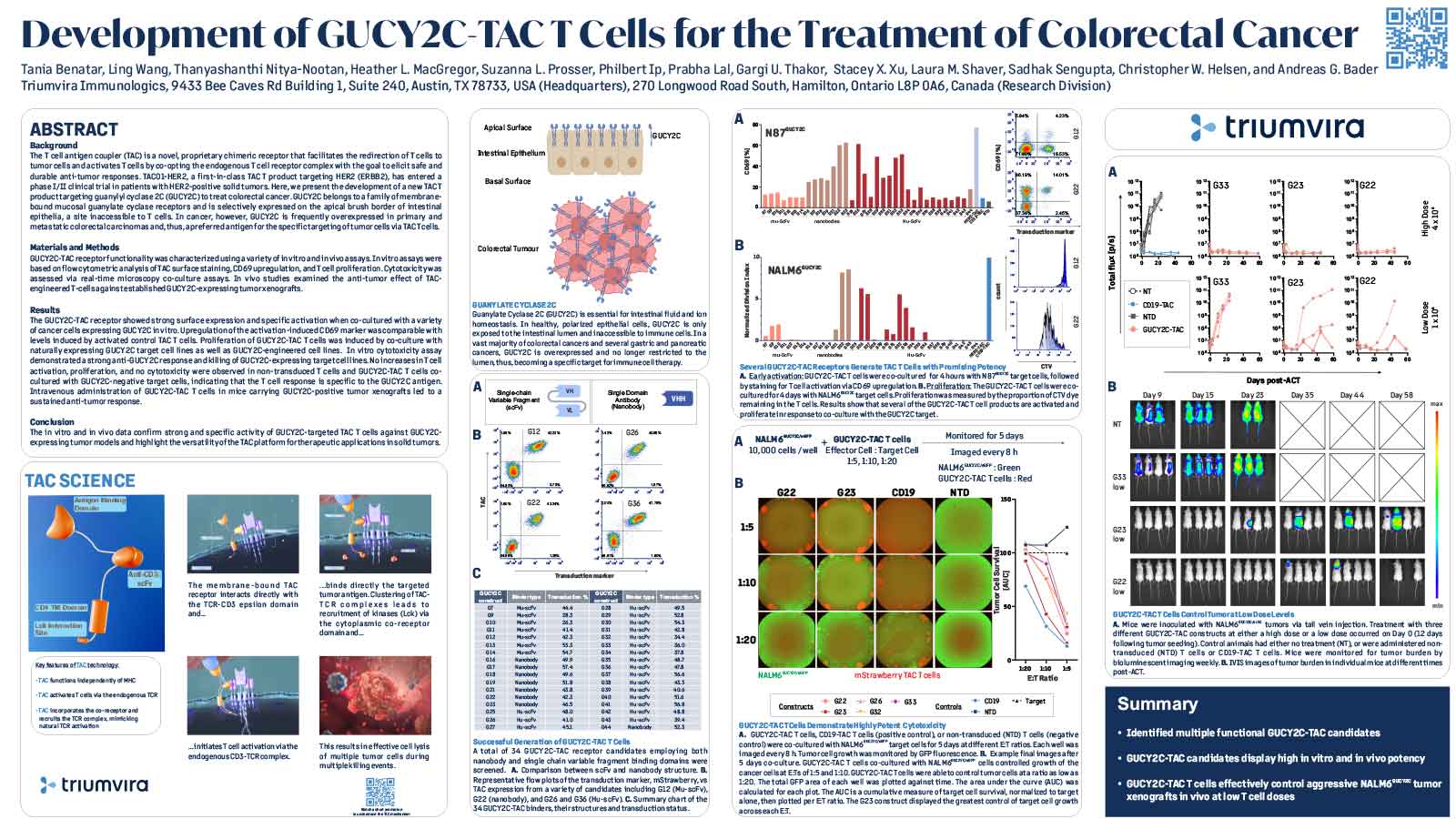
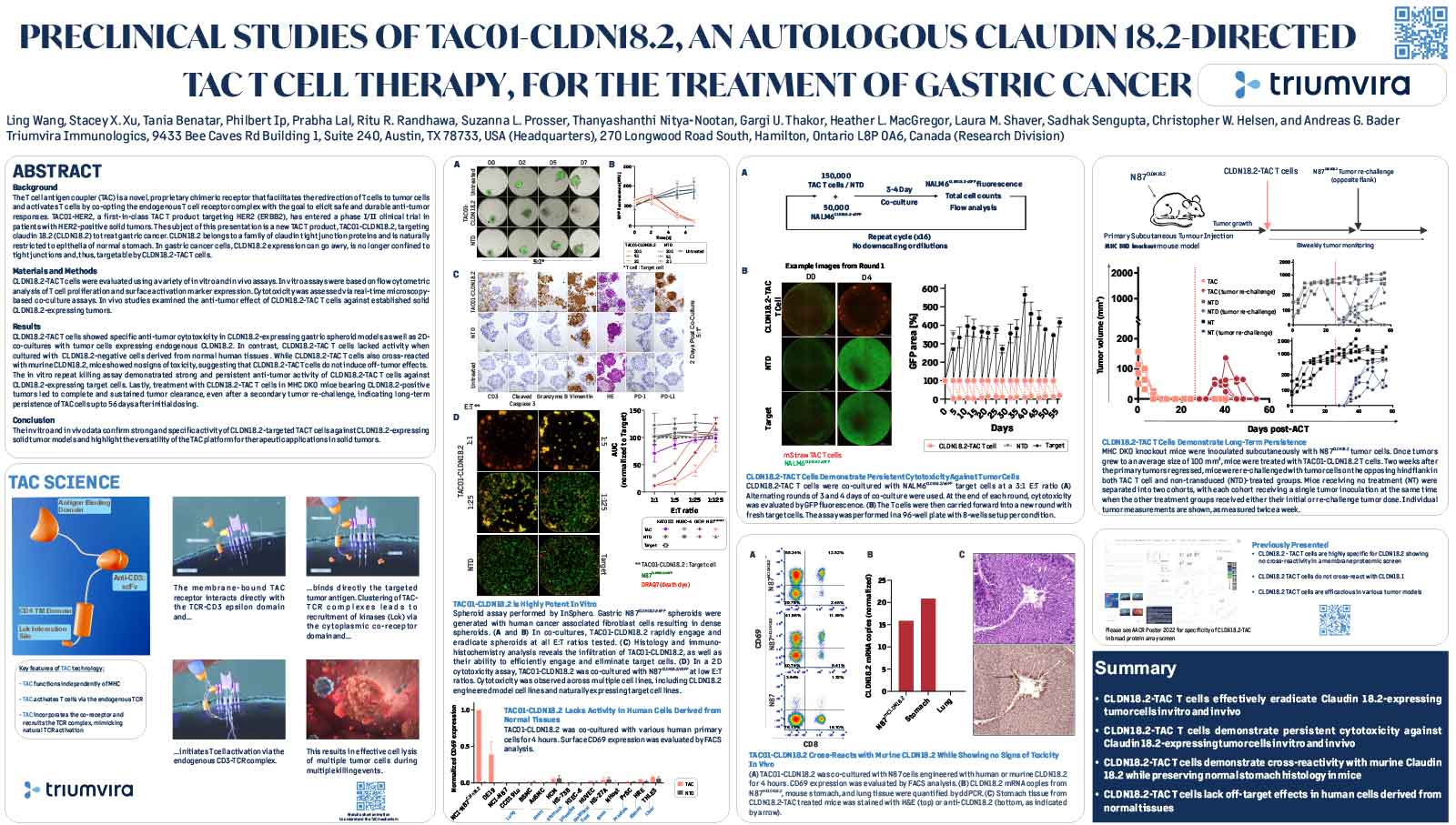
T cell antigen coupler (TAC) is a chimeric receptor that redirects T cells (TAC-T) towards surface-expressed tumor antigens to create safe and durable anti-cancer immune responses. The TAC activates T cells by co-opting the endogenous T cell receptor machinery via a CD3ε-specific binding motif and a cytoplasmic co-receptor tail. TAC01-HER2, a first-in-class TAC-T product targeting HER2 (ERBB2), has entered a phase I/II clinical trial. Here, we have characterized the fate of TAC-T cells during anti-tumor responses in vitro and in vivo.
Materials and Methods
In vitro, HER2-specific TAC-T products were challenged with HER2-expressing and HER2-negative tumor cells. Kinetics of T cell proliferation, degranulation, activation, differentiation, and memory generation was assessed by flow cytometry. TAC-T products were subjected to multiple rounds of tumor cell exposure in vitro to test the durability of the Tcell-mediated immune response. Bioinformatic clustering analysis of flow cytometry data was performed to identify T cell populations and track them over time.T cell expansion in blood, tumor, bone marrow, and spleen was evaluated in vivo after primary xenograft tumor treatment and secondary tumor rechallenge. Tumor- and spleen-infiltrating or circulating T cells were phenotyped by flow cytometry after treatment with TAC-T cells.
Results
Co-culture studies revealed that TAC-T products become rapidly activated and degranulate upon contact with HER2-expressing, but not HER2-negative, cell lines. Activation coincided with rapid downregulation of the TAC receptor. A large proportion of the T cells expressed activation markers, and a majority of these also expressed degranulation markers, indicating ongoing cytotoxicity. In vitro and in vivo studies demonstrated a CD8-biased response characterized by a considerable expansion in the activated CD8 population enriched at the tumor site. Later, activation and differentiation markers returned to baseline concurrently with the re-emergence of surface TAC expression, initiating T cell proliferation. Importantly, central memory T cells were expanded, and stem-like cells were maintained, suggesting strong self-renewal potential. In vitro serial cytotoxicity assays showed that TAC-T products could repeatedly kill tumor cells up to 12 rounds over 40 days. In tumor rechallenge experiments, a single dose of TAC-T cells expanded to clear solid tumor xenografts and protected mice from a second tumor challenge 30 days post-initial tumor clearance, indicating long-lasting T cell persistence.
Conclusion
The TAC-T product mounts an effective anti-tumor response in multiple preclinical models, comprising activated TAC-T cells that do not become terminally exhausted but are dominated by an activated CD8 response and supported by the expansion of a memory population, indicating robust self-renewal capacity.
View/Download Poster