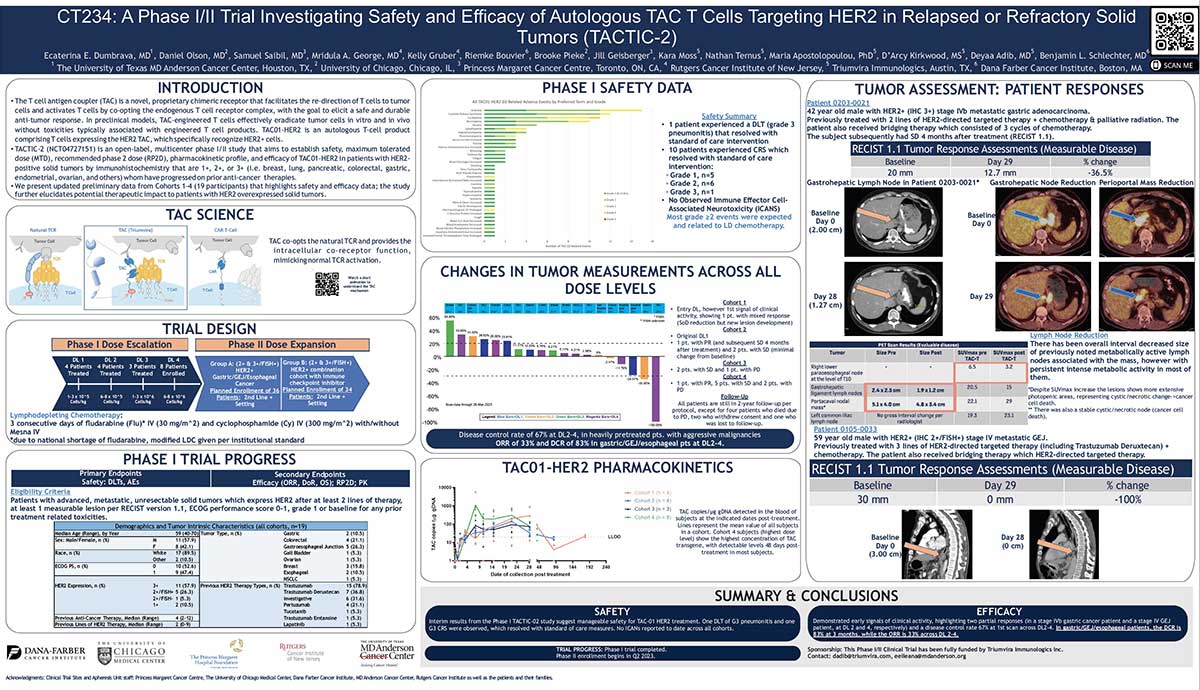
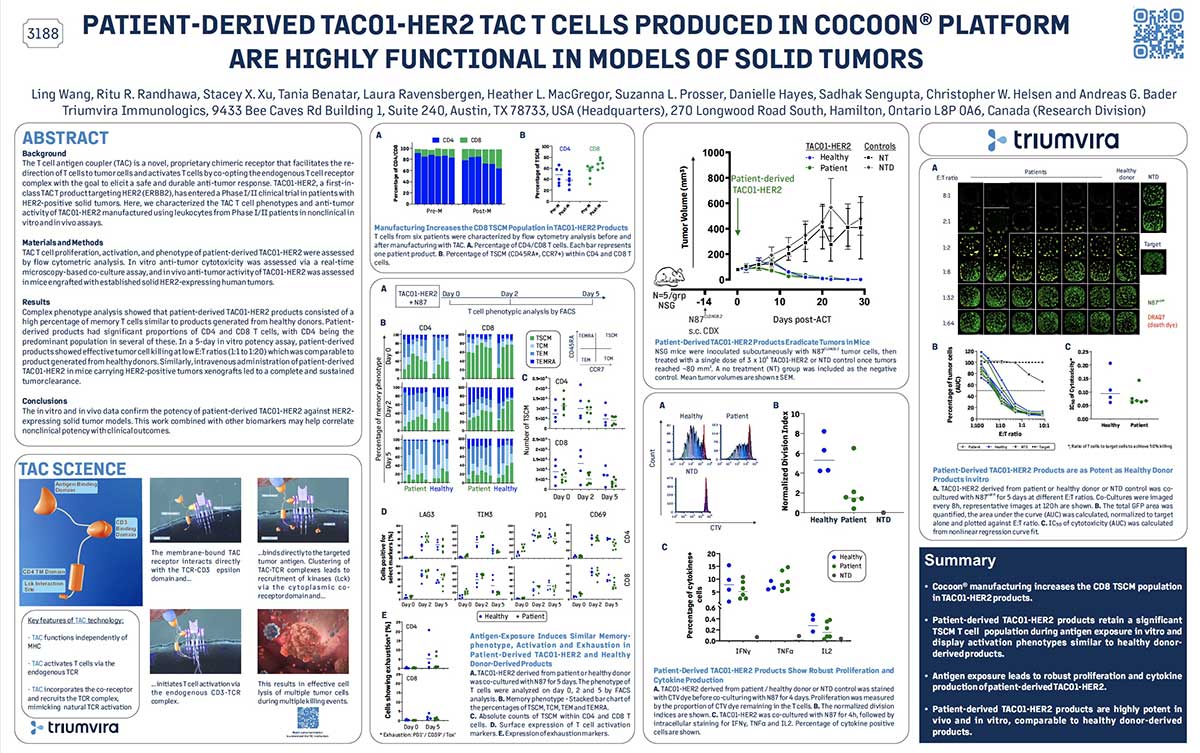
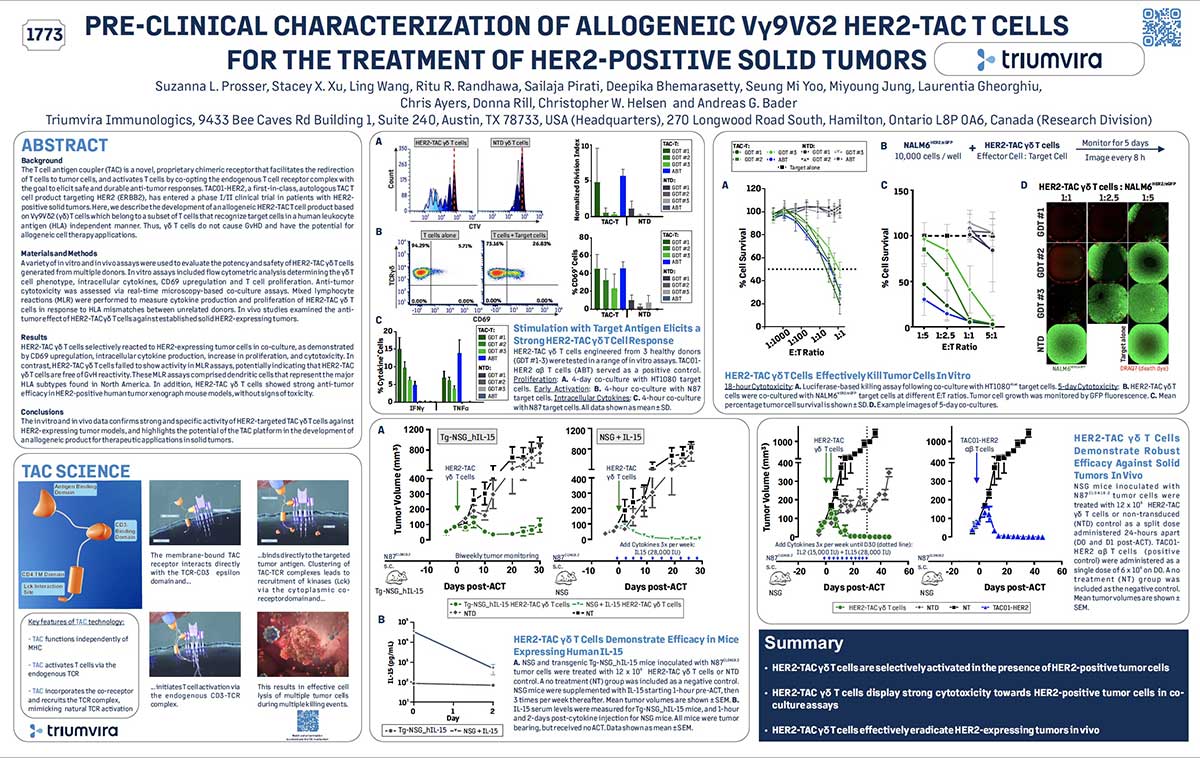
Join us in this episode as we talk with Paul Lammers, CEO of Triumvira Immunologics, a clinical-stage biopharmaceutical company focusing on developing T cell therapies for cancer treatment. Born in the Netherlands and now an American citizen, Lammers’ early life shaped his views on being bold and making connections with people a priority. He shares his insights on crucial but often neglected topics in life science leadership, while also revealing his joy as a father to three grown daughters and a grandfather.
We discuss the importance of transparent and open communication between leaders and employees, especially during times of change or uncertainty. Lammers also shares his thoughts on the challenges of balancing innovation and risk with regulatory and financial constraints. Additionally, we delve into the significance of investing in talent development and creating opportunities for employees to learn and grow, both for their own advancement and the success of the organization. Tune in to learn from Paul Lammers’ experiences and gain insights into effective leadership strategies in the biotech industry.