US-based Triumvira Immunologics will aim for accelerated approval for its TAC01-HER2 by the US Food and Drug Administration (FDA) in gastric and oesophageal cancers, chief medical officer Dr. Deyaa Adib told Clinical Trials Arena.
Adib noted: “It’s an area of significant unmet medical needs, where the standard of care has not changed over the last 40 years.”
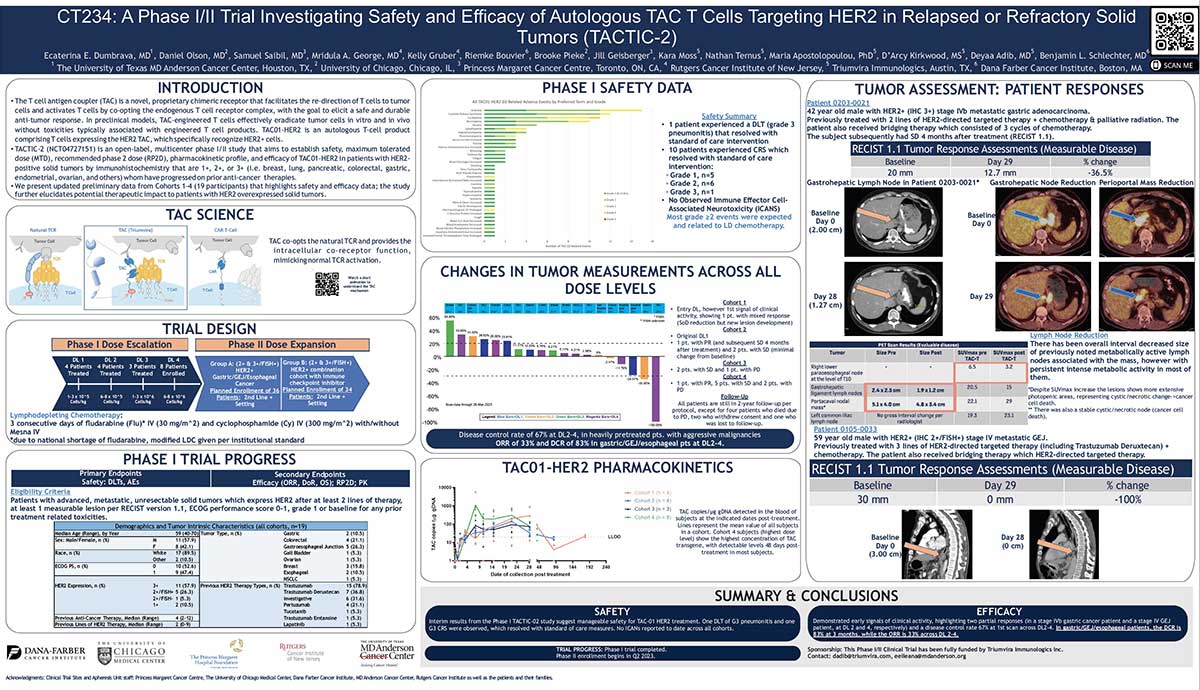
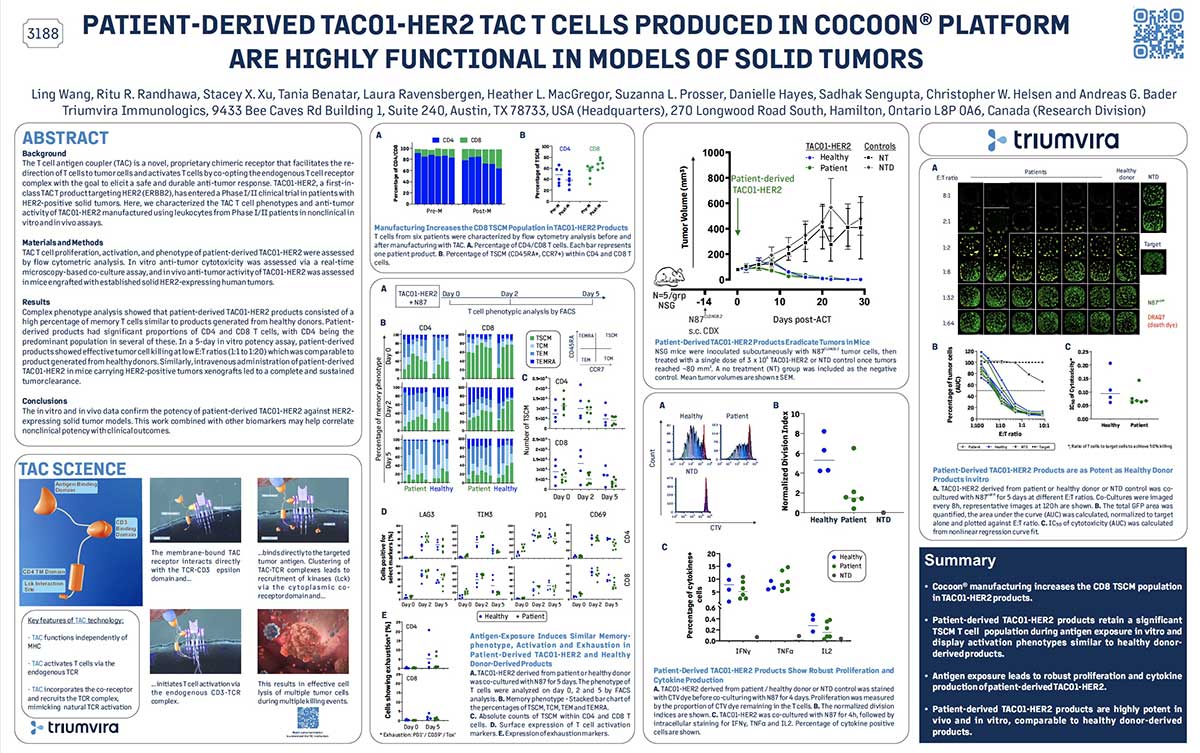
TAC01-HER2 is a novel cell therapy that consists of genetically engineered autologous T cells expressing T-cell antigen coupler (TAC), which recognizes human epidermal growth factor receptor 2 (HER2).
Triumvira Immunologics expects to complete the Phase II component of their Phase I/II trial in HER2-positive solid tumors in 2025. The Phase II portion will be a registration-enabling study and it is based on the three-stage Simon hypothesis. The second part of the trial is expected to enroll 72 patients who have passed between two to four prior lines.