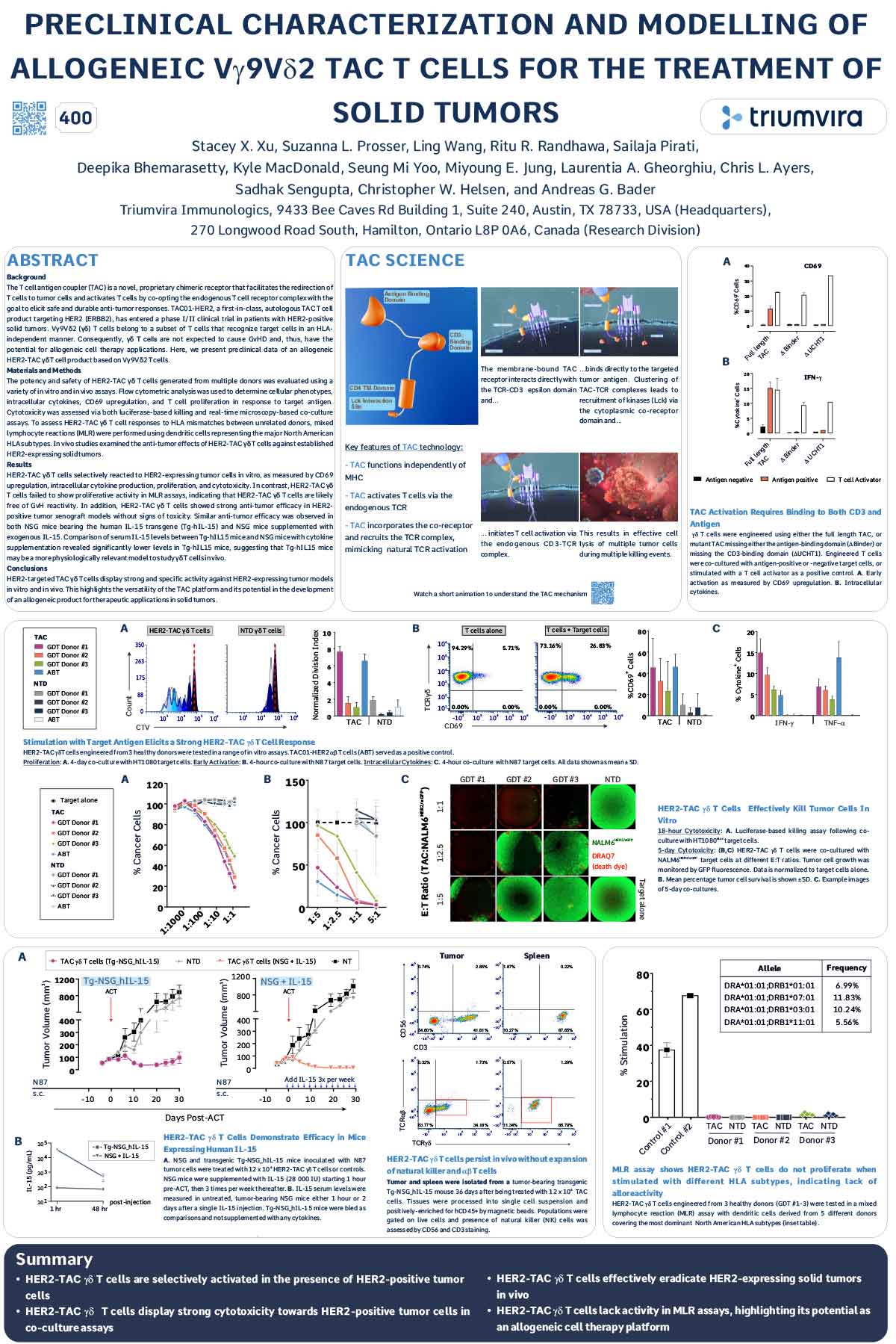
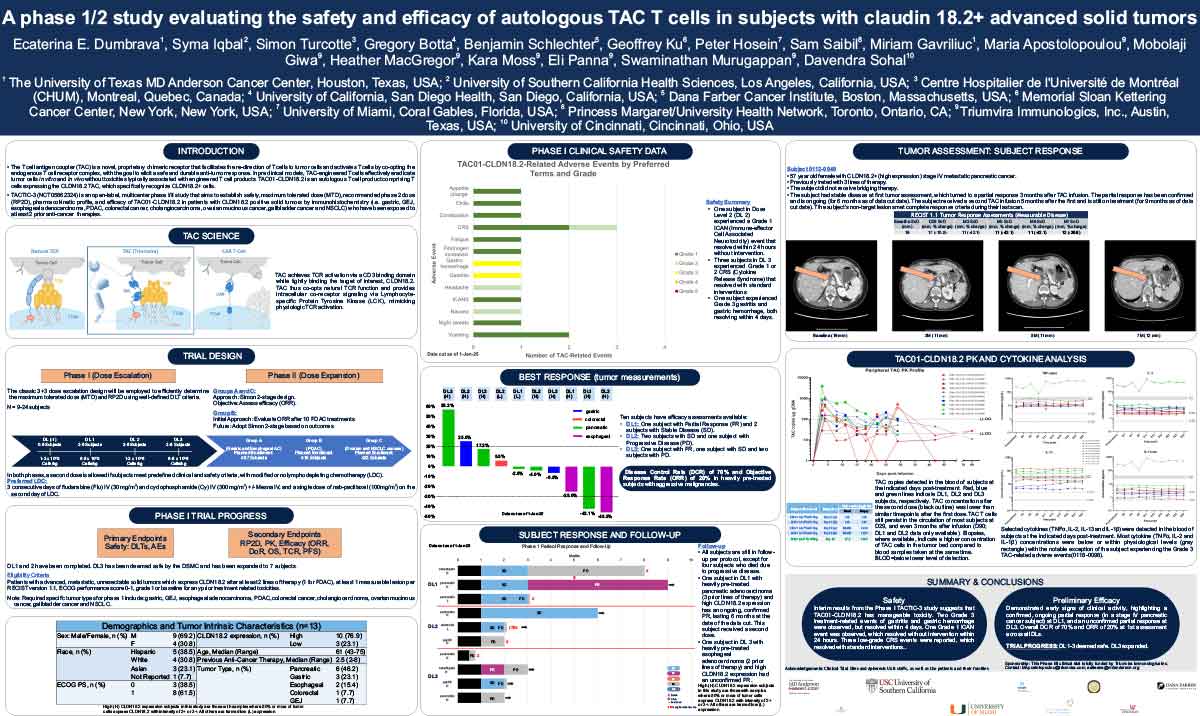
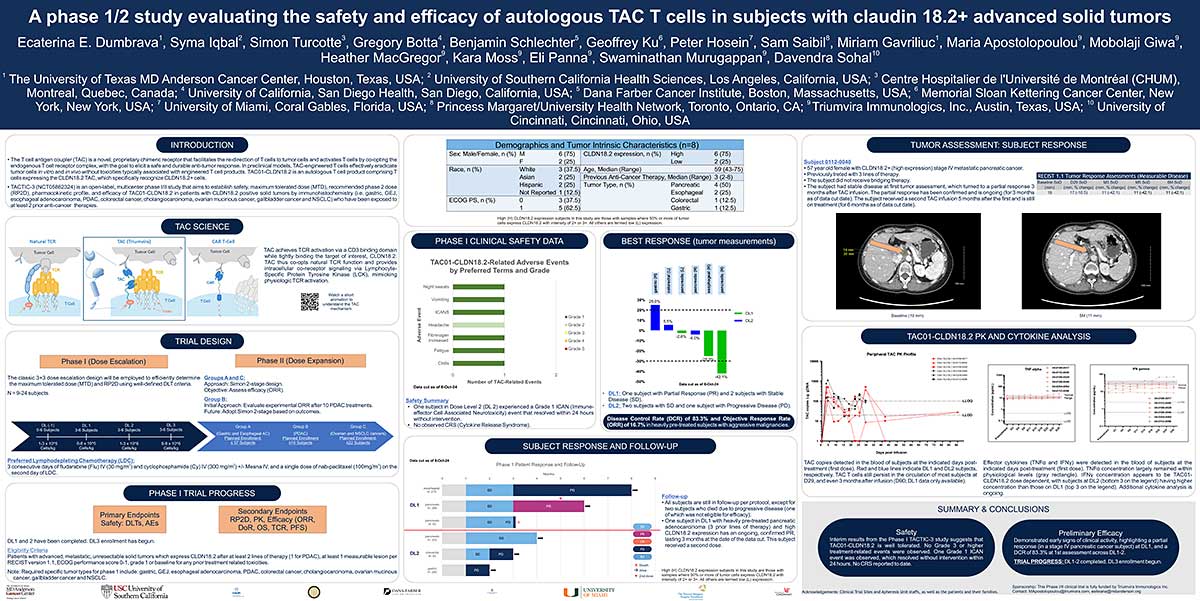
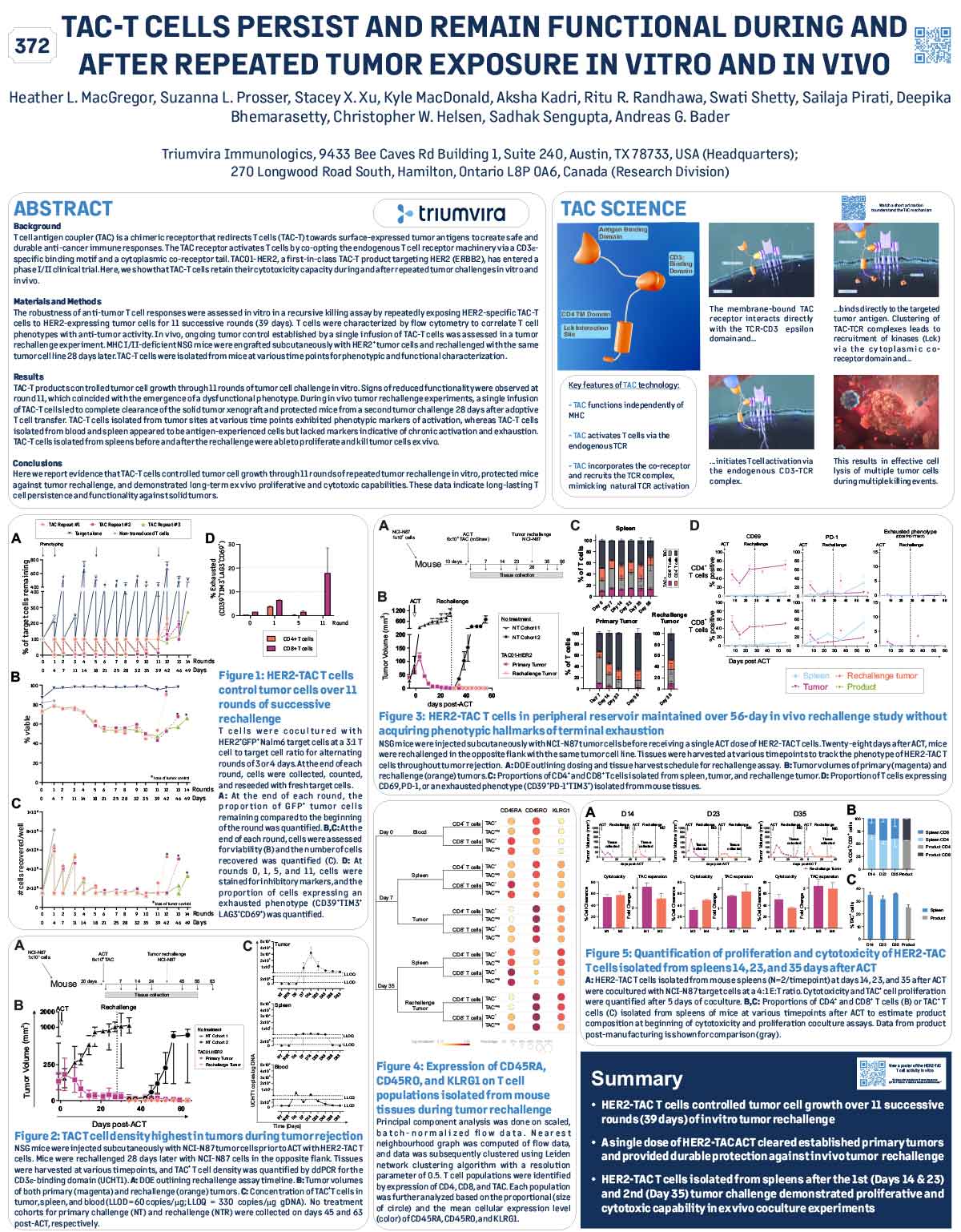
ABSTRACT
Background
The T cell antigen coupler (TAC) is a novel, proprietary chimeric receptor that facilitates the redirection of T cells to tumor cells and activates T cells by co-opting the endogenous T cell receptor complex with the goal to elicit safe and durable anti-tumor responses. TAC01-HER2, a first-in-class, autologous TAC T cell product targeting HER2 (ERBB2), has entered a phase I/II clinical trial in patients with HER2-positive solid tumors. Vγ9Vδ2 (γδ) T cells belong to a subset of T cells that recognize target cells in an HLAindependent manner. Consequently, γδ T cells are not expected to cause GvHD and, thus, have the potential for allogeneic cell therapy applications. Here, we present preclinical data of an allogeneic HER2-TAC γδ T cell product based on Vγ9Vδ2 T cells.
Materials and Methods
The potency and safety of HER2-TAC γδ T cells generated from multiple donors was evaluated using a variety of in vitro and in vivo assays. Flow cytometric analysis was used to determine cellular phenotypes, intracellular cytokines, CD69 upregulation, and T cell proliferation in response to target antigen. Cytotoxicity was assessed via both luciferase-based killing and real-time microscopy-based co-culture assays. To assess HER2-TAC γδ T cell responses to HLA mismatches between unrelated donors, mixed lymphocyte reactions (MLR) were performed using dendritic cells representing the major North American HLA subtypes. In vivo studies examined the anti-tumor effects of HER2-TAC γδ T cells against established HER2-expressing solid tumors.
Results
HER2-TAC γδ T cells selectively reacted to HER2-expressing tumor cells in vitro, as measured by CD69 upregulation, intracellular cytokine production, proliferation, and cytotoxicity. In contrast, HER2-TAC γδ T cells failed to show proliferative activity in MLR assays, indicating that HER2-TAC γδ T cells are likely free of GvH reactivity. In addition, HER2-TAC γδ T cells showed strong anti-tumor efficacy in HER2- positive tumor xenograft models without signs of toxicity. Similar anti-tumor efficacy was observed in both NSG mice bearing the human IL-15 transgene (Tg-hIL-15) and NSG mice supplemented with exogenous IL-15. Comparison of serum IL-15 levels between Tg-hIL15 mice and NSG mice with cytokine supplementation revealed significantly lower levels in Tg-hIL15 mice, suggesting that Tg-hIL15 mice may be a more physiologically relevant model to study γδ T cells in vivo.
Conclusions
HER2-targeted TAC γδ T cells display strong and specific activity against HER2-expressing tumor models in vitro and in vivo. This highlights the versatility of the TAC platform and its potential in the development of an allogeneic product for therapeutic applications in solid tumors.
View/Download Poster