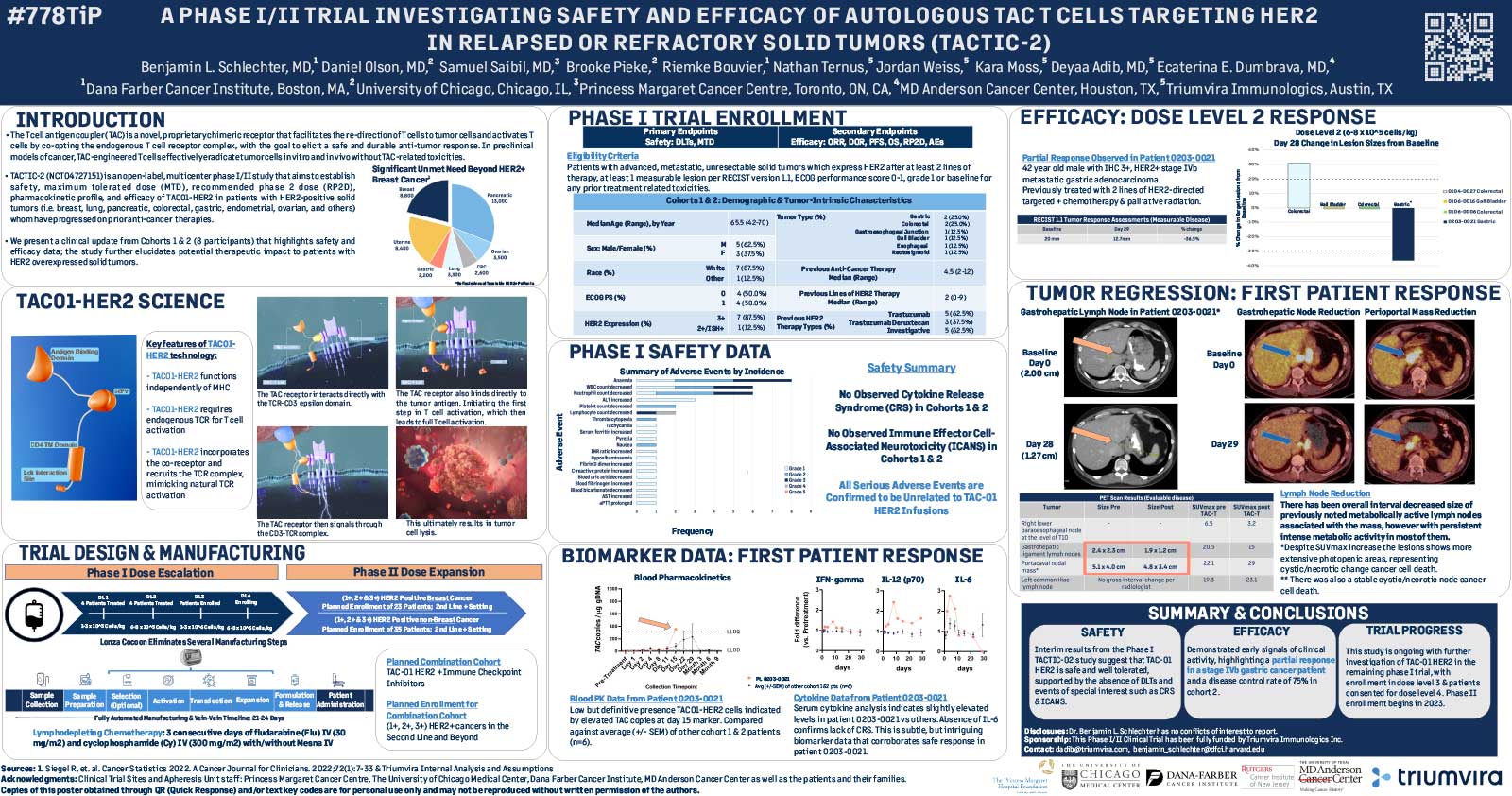
Early signals of clinical activity observed in second dosing cohort with one partial response TAC01-HER2 was safe and well-tolerated in the first two dosing cohorts of TACTIC-2 Phase 1/2 clinical trial
AUSTIN, Texas, and HAMILTON, Ontario, Sep. 12, 2022 – Triumvira Immunologics (“Triumvira”), a clinical-stage company developing novel, targeted autologous and allogeneic T cell therapeutics that co-opt the natural biology of T cells to treat patients with solid tumors, today announced positive initial clinical data from its ongoing TACTIC‑2 Phase 1/2 trial of TAC01-HER2 in patients with human epidermal growth factor receptor 2 (HER2) positive solid tumors. Initial clinical data demonstrate TAC01-HER2 was well-tolerated in both dosing cohorts and early signals of clinical activity were observed in the higher of the two dosing cohorts, demonstrating a 75% disease control rate, including one partial response. These initial results were presented in a poster at the European Society for Medical Oncology (ESMO) 2022 Congress.
“Every milestone achievement bolsters our confidence as we leverage our novel, versatile TAC platform to develop a T cell therapy that is less toxic than existing T cell therapies yet effective in killing target-bearing solid tumors”
The first two dosing cohorts of the trial enrolled eight patients with advanced, metastatic, unresectable HER2-positive solid tumors who had experienced up to two prior lines of therapy including HER2 targeted therapies. Early signals of clinical activity were observed in the second dosing cohort (6-8 x 105 cells/kg) with a disease control rate of 75%. A partial response was observed in a patient with stage IVb metastatic gastric cancer who was heavily pre-treated and defined as 3+ HER2 by immunohistochemistry (IHC). CT scans taken 29-days after dosing showed a 36.5% reduction in tumor size in target lesions compared to baseline and the size of numerous metabolically active lymph nodes associated with the mass decreased. Two patients with significant disease burden within the second cohort, one with colorectal cancer and one with gall bladder cancer, have been observed with stable disease with no change in tumor measurements compared to baseline.