CAR T cells are the most popular strategy to hit the solid tumor-selective target, but companies are using at least five other methods
By Danielle Golovin / BioCentury Staff Writer
June 23, 2022
CAR T cells are the most popular strategy to hit the solid tumor-selective target, but companies are using at least five other methods.
At least 17 cell therapy programs are targeting solid tumor-selective HER2, 10 of which are CAR T cells. Companies are also expressing HER2 CARs on other cell types such as NK cells, myeloid cells, and macrophages.
CAR T cell therapies have been highly effective against hematological malignancies, but solid tumors have been more challenging. Overcoming the major obstacles, including a lack of highly tumor-selective solid tumor targets, was a major theme at the American Association for Cancer Research (AACR) annual meeting in April.
HER2 is by far the most popular of 19 targets for solid tumor CAR T programs in the clinic, with at least seven. The well-validated target has also served as a testing ground for next-generation antibody therapies including antibody-drug conjugates and bispecifics.
The three other clinical cell therapies against HER2 are a TAC T cell from Triumvira Immunologics Inc., a CAR macrophage from Carisma Therapeutics Inc. and an antibody conjugated to an NK cell from Acepodia Inc.
Two of the clinical programs are in Phase I/II testing, while the rest are in Phase I.
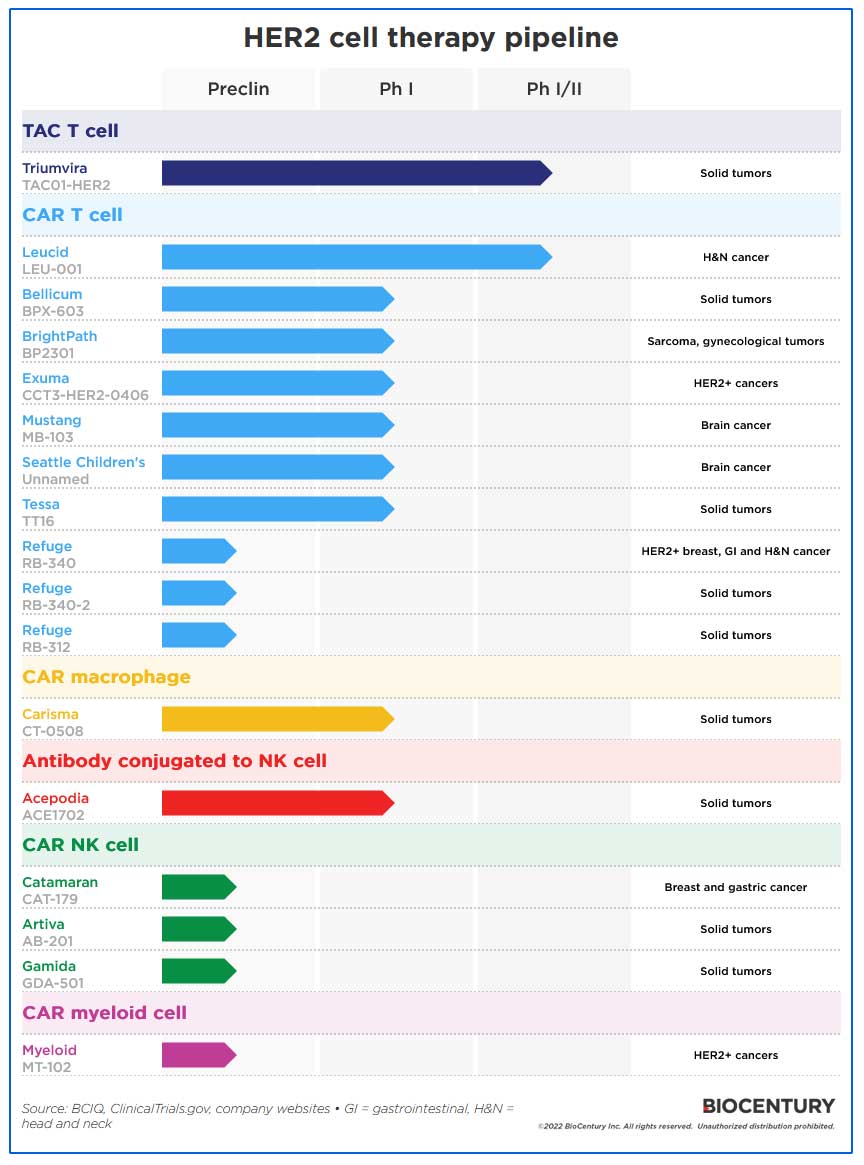
Triumvira’s TAC T cells are one of the most advanced programs. They incorporate a “T cell antigen coupler” receptor, a multidomain chimeric molecule that works directly with the cells’ natural T cell receptor (TCR) to help the cell recognize and attack cancer cells.
The company’s TAC01-HER2 cells have a TAC receptor comprising a HER2 binding domain, a central CD3 binding domain that interacts with the TCR, and CD4 co-receptor intracellular domain that anchors the TAC in the cell membrane and either activates or silences the T cell depending on the presence of a cancer antigen.
The other program in Phase I/II testing is CAR T cell LEU-001 from Leucid Bio Ltd. Its CAR construct recognizes eight distinct molecular targets according to its website, which includes homo- and heterodimers formed by the ErbB receptors, including HER2. The company has treated 18 patients with head and neck cancer, and 10 achieved stable disease.
At least six CAR T programs are in Phase I testing, and Refuge Biotechnologies Inc. has three preclinical assets, each with different gene modifications.
Other cell types with HER-targeting CARs include macrophages. Carisma believes using CAR macrophages solves the three major problems of CAR T therapies: insufficient trafficking into tumor cells, tumor antigen heterogeneity, and an immunosuppressive microenvironment. However, the company has so far provided little clinical data in support.
Acepodia is chemically conjugating mAbs to NK and other immune cells via complementary DNA linkers. Its first clinical product, ACE1702, incorporates anti-HER2 mAb trastuzumab.
The company reported interim Phase I data in September from a dose-escalation study with eight patients, showing one patient achieved a partial response at a dose of 3 billion cells per cycle.
Acepodia was founded in 2016 with global rights to components of the antibody-cell conjugation technology from Carolyn Bertozzi’s lab at the University of California Berkeley. The company told BioCentury that avoiding genetic engineering will lower its manufacturing costs.
Three companies have preclinical CAR NK cell programs, while Myeloid Therapeutics Inc. is pioneering CAR myeloid cells.


